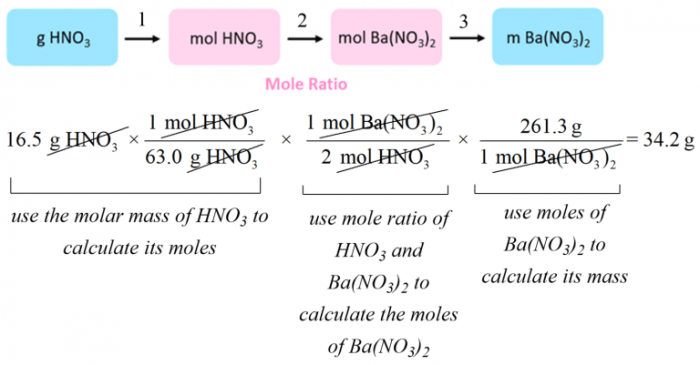
Fewer steps are required to solve stoichiometry problems when utilizing efficient methods, revolutionizing the problem-solving process. This streamlined approach not only simplifies calculations but also enhances accuracy and efficiency, leading to a deeper understanding of stoichiometry concepts.
By minimizing the number of steps involved in stoichiometry problems, students can focus on the core principles and develop a stronger grasp of the subject matter. This approach caters to diverse learning styles, making stoichiometry more accessible and engaging for all.
Fewer Steps in Stoichiometry Problem Solving: Fewer Steps Are Required To Solve Stoichiometry Problems When
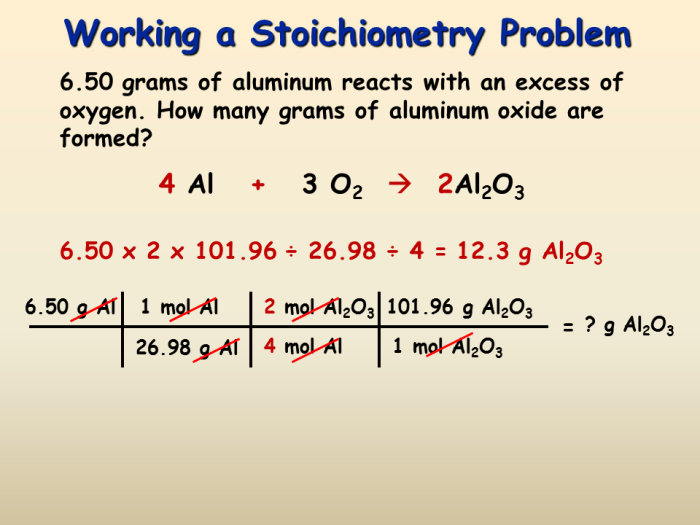
Requiring fewer steps to solve stoichiometry problems offers numerous benefits, including:
Benefits of Fewer Steps in Stoichiometry Problem Solving
- Simplified problem-solving: Reducing steps makes calculations less complex and easier to understand.
- Improved accuracy: With fewer steps, there are fewer opportunities for errors.
- Increased efficiency: Solving problems with fewer steps saves time and allows for more efficient use of resources.
Methods for Reducing Steps
Several techniques can minimize the number of steps in stoichiometry problems:
- Dimensional analysis: This method involves converting units and using dimensional analysis to simplify calculations.
- Mole ratios: Using mole ratios directly relates the number of moles of reactants and products, reducing the need for multiple conversions.
- Organizing calculations: Clearly organizing calculations and avoiding unnecessary conversions can streamline problem-solving.
Applications in Different Stoichiometry Concepts
Fewer steps benefit stoichiometry calculations in various concepts:
- Limiting reactants: Identifying the limiting reactant becomes easier when calculations are simplified.
- Empirical and molecular formulas: Determining empirical and molecular formulas requires fewer conversions and calculations.
- Gas stoichiometry: Solving gas stoichiometry problems involving gas laws and partial pressures is simplified with fewer steps.
Comparison with Traditional Methods
Using fewer steps is more efficient than traditional stoichiometry problem-solving methods:
- Time savings: Fewer steps significantly reduce the time required to solve problems.
- Error reduction: With fewer calculations, the potential for errors is minimized.
- Traditional methods: Traditional methods may still be preferred in complex problems or when accuracy is critical.
Impact on Student Learning, Fewer steps are required to solve stoichiometry problems when
Reducing steps enhances student understanding of stoichiometry concepts:
- Improved understanding: Fewer steps make it easier for students to grasp the underlying principles of stoichiometry.
- Different learning styles: Students with different learning styles benefit from simplified problem-solving methods.
- Incorporating fewer steps: Strategies for incorporating fewer steps into stoichiometry can be shared with students.
FAQ Guide
What are the benefits of using fewer steps in stoichiometry problem-solving?
Fewer steps simplify calculations, enhance accuracy, improve efficiency, and promote conceptual understanding.
How can I reduce the number of steps in stoichiometry problems?
Utilize dimensional analysis, mole ratios, and organize calculations to minimize unnecessary conversions.
How does using fewer steps impact student learning?
It enhances understanding, accommodates diverse learning styles, and fosters problem-solving proficiency.