Which statement compares the masses of two subatomic particles? This question delves into the realm of particle physics, where the fundamental building blocks of matter are meticulously studied. By comparing the masses of subatomic particles, scientists gain insights into the structure of matter and the forces that govern the universe.
Experimental techniques, such as mass spectrometry and particle accelerators, have enabled researchers to measure the masses of subatomic particles with remarkable precision. These comparisons have revealed fundamental properties of particles, including their charge, spin, and interactions with other particles.
1. Introduction

The masses of subatomic particles are fundamental properties that play a crucial role in understanding the structure of matter and the behavior of the universe. By comparing the masses of different subatomic particles, scientists can gain insights into the fundamental forces that govern their interactions and the nature of the universe.
Comparing the masses of subatomic particles has led to significant advancements in our understanding of the atom, the nucleus, and the elementary particles that make up all matter. It has also contributed to the development of new technologies, such as particle accelerators and nuclear reactors.
2. Methods for Comparing the Masses of Subatomic Particles
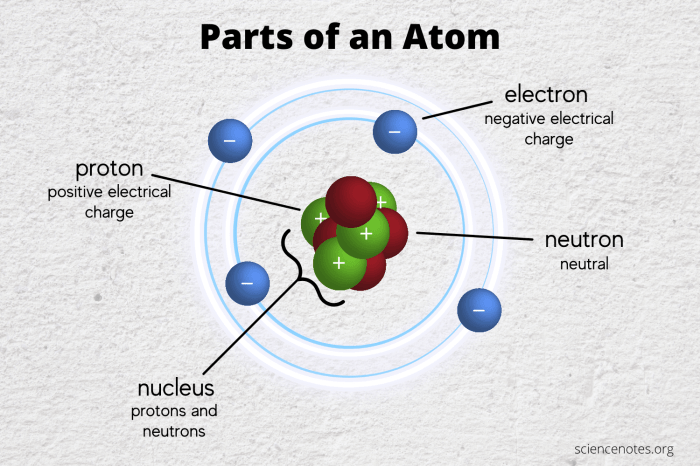
The masses of subatomic particles are typically measured using a variety of experimental techniques, including:
- Mass spectrometry: This technique separates ions by their mass-to-charge ratio, allowing for the precise determination of the mass of individual ions.
- Particle accelerators: These devices accelerate charged particles to high energies, and the resulting collisions can be used to measure the masses of the particles involved.
- Nuclear reactions: By studying the products of nuclear reactions, scientists can infer the masses of the particles involved.
Each of these techniques has its own limitations and uncertainties, and the choice of technique depends on the specific particles being studied and the desired level of precision.
3. Examples of Comparisons of the Masses of Subatomic Particles
One of the most well-known examples of comparing the masses of subatomic particles is the comparison of the masses of the proton and the neutron. These particles have nearly identical masses, but the neutron is slightly heavier than the proton.
This difference in mass is due to the presence of an additional down quark in the neutron.
Another example is the comparison of the masses of the electron and the muon. The muon is a subatomic particle that is very similar to the electron, but it is much heavier. This difference in mass is due to the presence of an additional pair of quarks in the muon.
4. Applications of Comparing the Masses of Subatomic Particles: Which Statement Compares The Masses Of Two Subatomic Particles
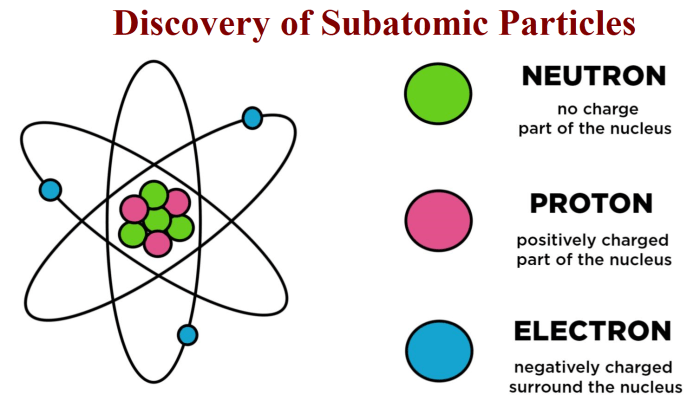
Comparing the masses of subatomic particles has a wide range of applications in fields such as nuclear physics and particle physics. These applications include:
- Nuclear physics: The masses of subatomic particles are used to calculate the binding energy of nuclei and to understand the stability of different isotopes.
- Particle physics: The masses of subatomic particles are used to test theories of particle physics and to search for new particles.
- Astrophysics: The masses of subatomic particles are used to understand the evolution of stars and the formation of galaxies.
FAQ Summary
What is the significance of comparing the masses of subatomic particles?
Comparing the masses of subatomic particles provides valuable insights into their fundamental properties, such as charge, spin, and interactions with other particles. It also helps scientists understand the structure of matter and the forces that govern the universe.
How are the masses of subatomic particles measured?
The masses of subatomic particles are measured using experimental techniques such as mass spectrometry and particle accelerators. These techniques allow scientists to determine the mass-to-charge ratio of particles, which can then be used to calculate their mass.
What are some examples of subatomic particles whose masses have been compared?
Examples of subatomic particles whose masses have been compared include protons, neutrons, electrons, and quarks. These comparisons have contributed to our understanding of the composition of atoms and the structure of the nucleus.