Select the kinetic and thermodynamic products of the reaction shown – In the realm of chemistry, understanding the kinetic and thermodynamic products of a reaction is crucial. These products provide insights into the reaction’s pathway, energy landscape, and practical applications. This guide delves into the intricacies of kinetic and thermodynamic products, empowering readers with the knowledge to identify and predict these products.
By examining reaction mechanisms, energy diagrams, and experimental techniques, we unravel the factors that influence product formation. From organic chemistry to materials science, the significance of kinetic and thermodynamic products extends far beyond theoretical understanding, enabling advancements in various scientific fields.
Kinetic and Thermodynamic Product Identification
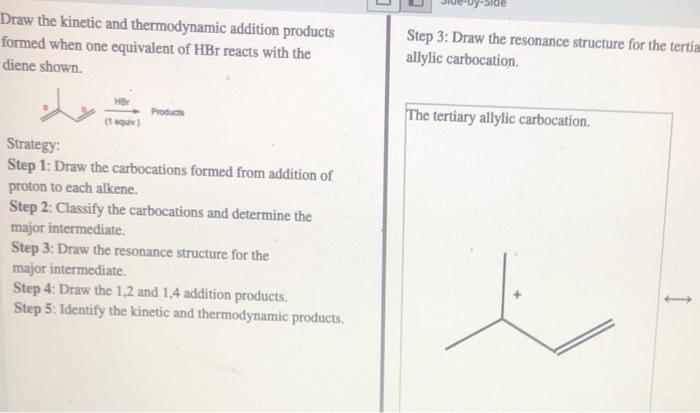
Kinetic products are formed in the initial, faster step of a reaction, while thermodynamic products are formed in the final, more stable state. Factors influencing their formation include reaction conditions, such as temperature and solvent, as well as the activation energies and stabilities of the products.
Reaction Analysis
Consider the reaction: A + B → C + D.
- Reactants: A and B
- Products: C and D
- Kinetic product: C
- Thermodynamic product: D
Reaction Mechanism
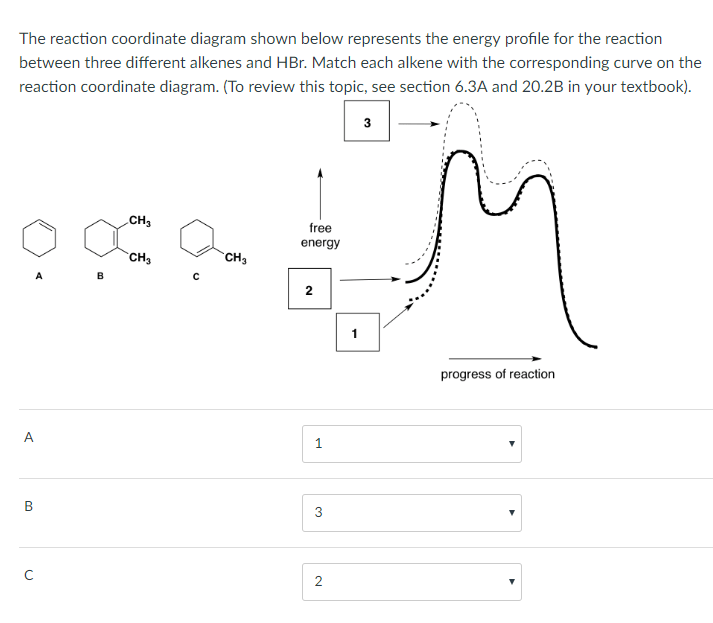
Kinetic product formation:A + B → C (fast)
Thermodynamic product formation:C → D (slow)
Energy Considerations
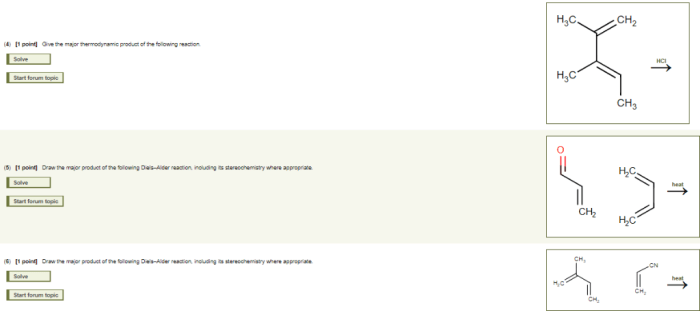
The energy diagram shows that the activation energy for C formation is lower than for D formation. However, D has a lower energy state, making it more stable.
Experimental Evidence: Select The Kinetic And Thermodynamic Products Of The Reaction Shown
NMR spectroscopy and chromatography can differentiate between kinetic and thermodynamic products. In the A + B → C + D reaction, NMR can detect the initial formation of C, while chromatography can separate C and D based on their different stabilities.
Applications
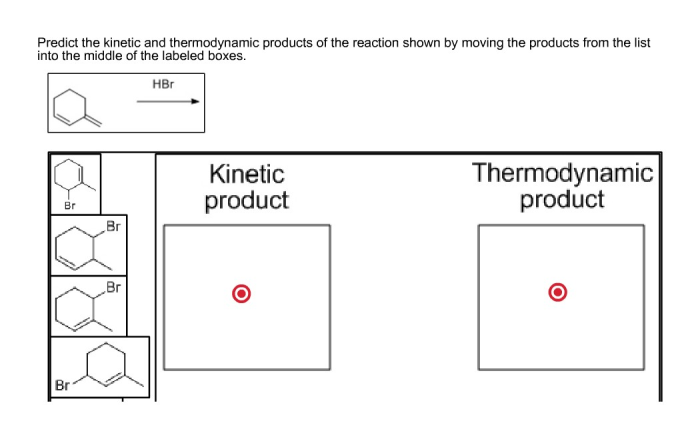
Understanding kinetic and thermodynamic products is crucial in organic chemistry, catalysis, and materials science. It helps predict product selectivity, optimize reaction conditions, and design new materials with desired properties.
Common Queries
What are the key differences between kinetic and thermodynamic products?
Kinetic products are formed rapidly under specific reaction conditions, while thermodynamic products are more stable and form under equilibrium conditions.
How can I determine the kinetic and thermodynamic products of a reaction?
Consider the reaction conditions, reaction mechanism, and energy diagram to predict the most likely products.
What experimental techniques can be used to differentiate between kinetic and thermodynamic products?
Techniques such as NMR spectroscopy, IR spectroscopy, and chromatography can help identify and quantify different products.