The heating curve for water worksheet presents a comprehensive analysis of the thermal behavior of water, unraveling the intricate physical and chemical transformations that occur as it transitions through various temperature ranges. This in-depth resource empowers students and researchers alike to gain a profound understanding of water’s unique properties and their implications in diverse scientific fields.
Delving into the intricacies of the heating curve, we embark on a journey to decipher the factors that influence its shape and position, unlocking the secrets behind water’s remarkable adaptability. Moreover, we explore the practical applications of heating curves, demonstrating their invaluable role in determining water purity, studying phase transitions, and optimizing heating and cooling processes.
Heating Curve of Water
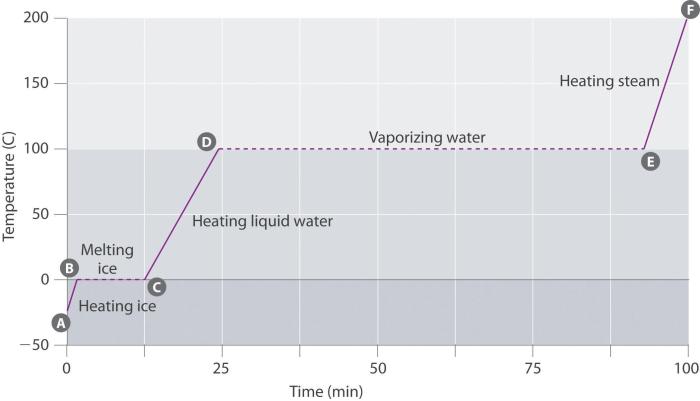
A heating curve is a graphical representation of the temperature change of a substance as it is heated or cooled. The heating curve for water is a particularly important one because water is such a common substance and because it undergoes several phase changes as it is heated or cooled.
These phase changes are associated with changes in the physical and chemical properties of water.
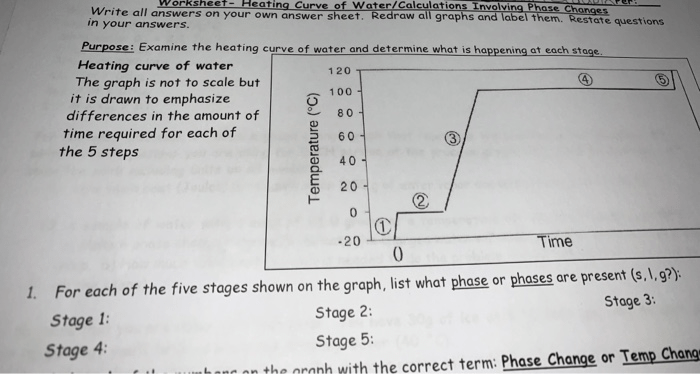
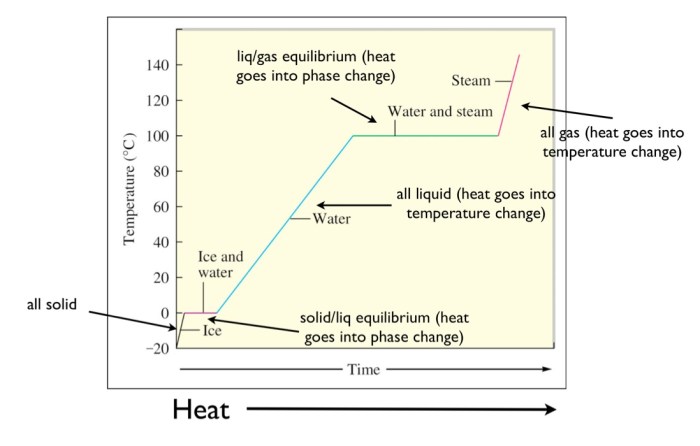
The heating curve for water can be divided into four distinct stages:
- Solid phase:In this stage, water is in the solid phase, and its temperature increases as it is heated. The slope of the heating curve in this stage is relatively low because the energy being added to the water is used to increase the kinetic energy of the water molecules, rather than to cause a phase change.
- Melting:At the melting point, the water begins to melt, and the temperature remains constant until all of the water has melted. The energy being added to the water during this stage is used to overcome the intermolecular forces that hold the water molecules together in the solid phase.
- Liquid phase:In this stage, water is in the liquid phase, and its temperature increases as it is heated. The slope of the heating curve in this stage is relatively high because the energy being added to the water is used to increase the kinetic energy of the water molecules, as well as to break some of the intermolecular bonds between the water molecules.
- Boiling:At the boiling point, the water begins to boil, and the temperature remains constant until all of the water has boiled away. The energy being added to the water during this stage is used to overcome the intermolecular forces that hold the water molecules together in the liquid phase.
Factors Affecting the Heating Curve, Heating curve for water worksheet
The shape and position of the heating curve for water can be affected by a number of factors, including:
- Temperature:The temperature of the water will affect the rate at which it heats up or cools down. The higher the temperature, the faster the water will heat up or cool down.
- Pressure:The pressure of the water will affect the boiling point of the water. The higher the pressure, the higher the boiling point.
- Volume:The volume of the water will affect the amount of time it takes to heat up or cool down. The larger the volume of water, the longer it will take to heat up or cool down.
- Impurities:The presence of impurities in the water will affect the heating curve. Impurities can lower the boiling point of the water and can also cause the water to freeze at a lower temperature.
Applications of Heating Curves
Heating curves can be used for a variety of practical applications, including:
- Determining the purity of water:The presence of impurities in water can be detected by analyzing the heating curve of the water. Impurities will cause the heating curve to deviate from the ideal heating curve for pure water.
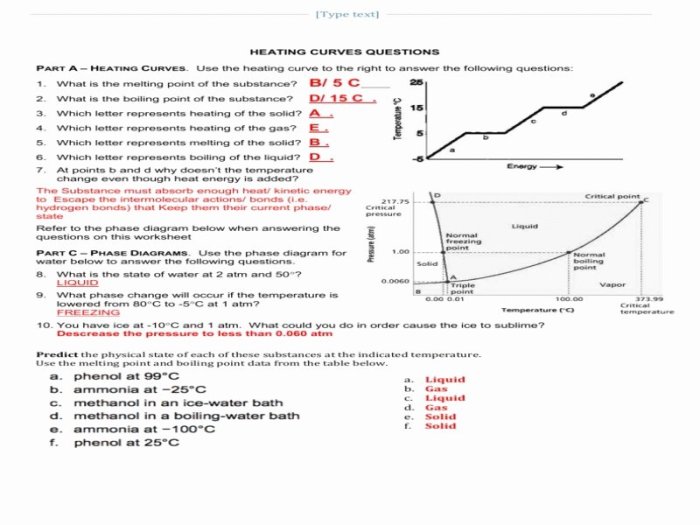
- Studying phase transitions:Heating curves can be used to study the phase transitions of water. The heating curve can be used to determine the melting point and boiling point of water, as well as the enthalpy changes associated with these phase transitions.
- Optimizing heating and cooling processes:Heating curves can be used to optimize heating and cooling processes. By understanding the heating curve of a substance, it is possible to determine the most efficient way to heat or cool the substance.
FAQ Overview: Heating Curve For Water Worksheet
What is a heating curve?
A heating curve is a graphical representation of the temperature change of a substance as it is heated at a constant rate.
What are the different stages of the heating curve for water?
The heating curve for water consists of four distinct stages: solid, liquid, boiling, and vapor.
What factors can affect the shape and position of the heating curve for water?
Factors that can affect the shape and position of the heating curve for water include pressure, volume, and impurities.