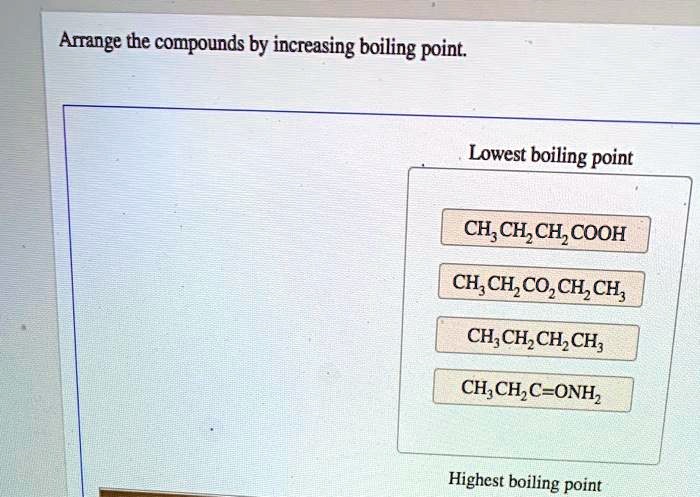
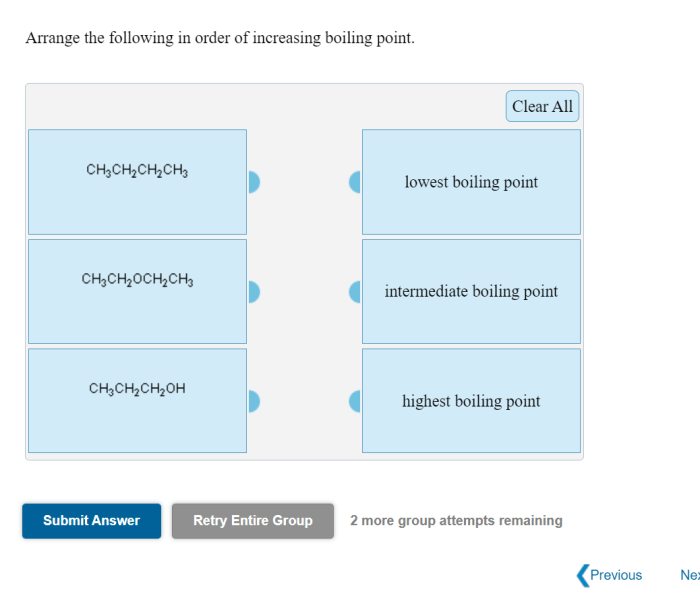
Arrange the compounds below in order of increasing boiling point. Boiling point is a crucial physical property that plays a significant role in various scientific disciplines. Understanding the factors influencing boiling point and the methods used to arrange compounds accordingly is essential for effective chemical analysis, process optimization, and material characterization.
This comprehensive guide delves into the concept of boiling point, explores the key factors affecting it, and discusses the various methods employed to arrange compounds in order of increasing boiling point. Practical examples and applications of this knowledge are also presented, highlighting its importance in fields such as chemical engineering, pharmaceutical development, and environmental science.
Boiling Point of Compounds: Arrange The Compounds Below In Order Of Increasing Boiling Point.
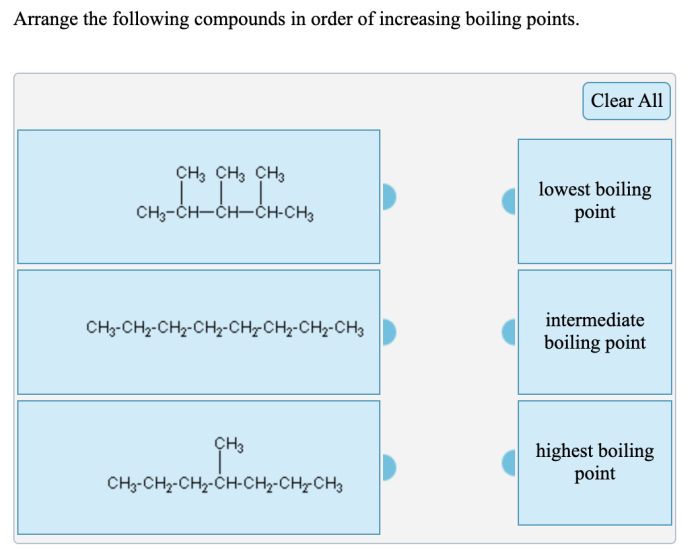
The boiling point of a compound is the temperature at which it transitions from a liquid to a gas. Arranging compounds in order of increasing boiling point is essential for understanding their physical properties and behavior.
Factors Affecting Boiling Point
- Molecular weight:Heavier molecules have stronger intermolecular forces, resulting in higher boiling points.
- Intermolecular forces:Compounds with stronger intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, have higher boiling points.
- Surface area:Compounds with larger surface areas have weaker intermolecular forces, leading to lower boiling points.
Methods for Arranging Compounds, Arrange the compounds below in order of increasing boiling point.
- Structural analysis:The molecular structure of a compound can provide insights into its intermolecular forces and boiling point.
- Experimental measurements:Boiling points can be experimentally determined using various techniques, such as ebulliometry or differential scanning calorimetry.
- Computational modeling:Molecular simulations can predict the boiling points of compounds based on their molecular properties.
Examples and Applications
- The following compounds are arranged in order of increasing boiling point: methane, ethanol, water, benzene, and hexane.
- This knowledge is used in chemical engineering for process design, in the pharmaceutical industry for drug formulation, and in environmental science for understanding pollutant behavior.
Limitations and Considerations
Arranging compounds based on boiling point assumes that intermolecular forces are the dominant factor influencing boiling point. However, other factors, such as molecular shape and polarity, can also play a role.
Questions Often Asked
What is the significance of boiling point in chemistry?
Boiling point is a key indicator of a compound’s volatility, stability, and intermolecular interactions. It provides valuable information for chemical synthesis, purification, and various industrial processes.
How can we experimentally determine the boiling point of a compound?
Experimental methods such as ebulliometry and differential scanning calorimetry (DSC) are commonly used to accurately measure the boiling point of a compound.
What are the limitations of using boiling point as a sole criterion for compound identification?
While boiling point can be a useful property for compound identification, it should be used in conjunction with other analytical techniques, as compounds with similar boiling points may have different structures or properties.